Basics of Matter

Matter is the universe’s fundamental building block, permeating all aspects of our existence. As we observe our environment, we come across a wide variety of objects and find out about the physical nature of matter, each identified by its shape, size, and texture. Despite their diversity, these phenomena are all types of matter, implying a fundamental unity. Everything, from the air we breathe to the food we consume, from the solid ground beneath our feet to the celestial bodies that fill the entire sky, is complexly constructed from the structure of matter.
We began this journey to unravel the secrets of matter and investigate its nature and properties. Through this detailed examination, we want to unearth the secrets concealed within matter and better understand its significance. By delving into the essence of matter, we have access to a world of knowledge that exposes the fundamental nature of existence.
Early Classifications
Throughout history, humans have attempted to understand their surroundings, resulting in many different kinds of matter. Early Indian and Greek philosophers proposed elemental groupings, such as the “Panch Tatva,” which included air, earth, fire, sky, and water. These classifications established the foundation for modern scientific understanding, which classifies matter based on its physical and chemical properties.
Read More From Science
Physical Nature of Matter
Classification of matter based on physical nature:
- Matter is Made Up of Particles
- How small are these particles of matter?
Matter is Made Up of Particles
For thousands of years, there has been discussion over the fundamental nature of matter—whether it exists as one unit or is composed of individual particles. Scientists have created evidence to support their theory that matter is made up of separate particles by dissolving items in water. These experiments demonstrate how molecules break down into smaller particles when dissolved, implying the presence of different matter particles. This concept of substance as a collection of particles has far-reaching implications for many fields of study, and it continues to influence our knowledge of the world on both a macroscopic and microscopic scale.
Example: Dissolving Salt in Water

When we dissolve salt in water, the salt particles spread out and fill the spaces between the water particles. This occurs because matter is composed of microscopic particles with gaps between them. As the salt dissolves, its particles separate and travel into the gaps, forming a homogenous mixture in which the salt is uniformly distributed throughout the water. This process demonstrates the concept of interparticle gaps in matter, demonstrating how substances can interact at the molecular level.
Exploring Particles Size
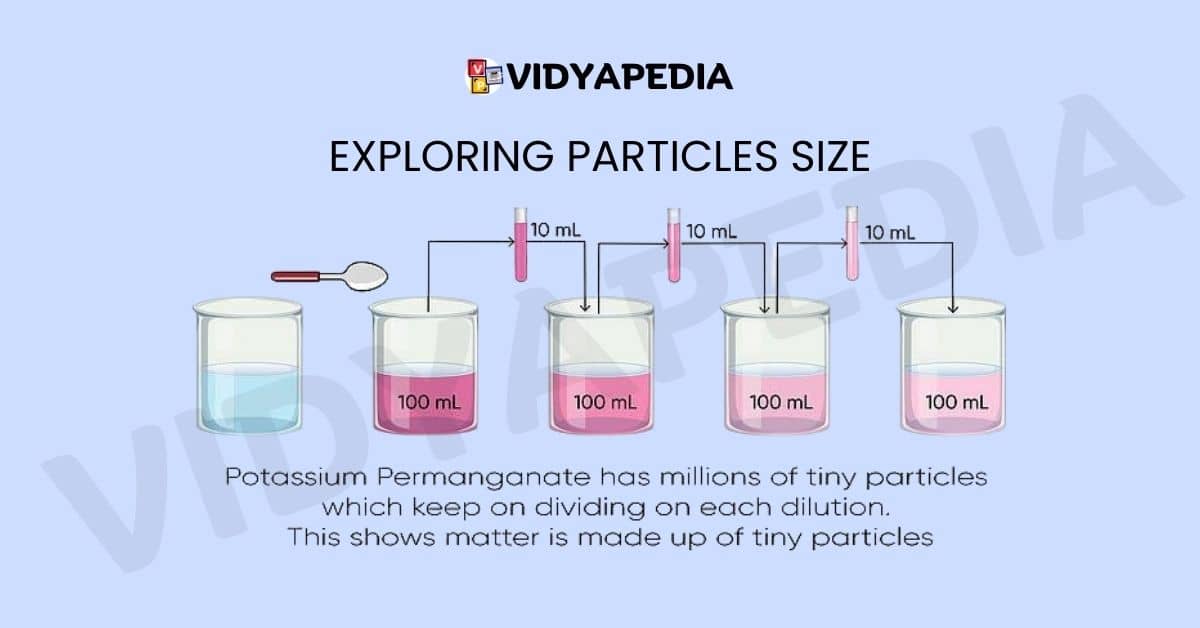
Experiments with chemicals such as potassium permanganate provide unique views on the microscopic size of matter particles. Surprisingly, only a few crystals of potassium permanganate can color a large volume of water. This phenomenon shows how many tiny particles can exist inside a single crystal. Such experiments stretch the limits of our thinking and highlight the complexities of the tiny galaxy by emphasizing the extraordinarily small size of matter particles. These results contribute to our understanding of the deep and sophisticated nature of matter’s fundamental building blocks.
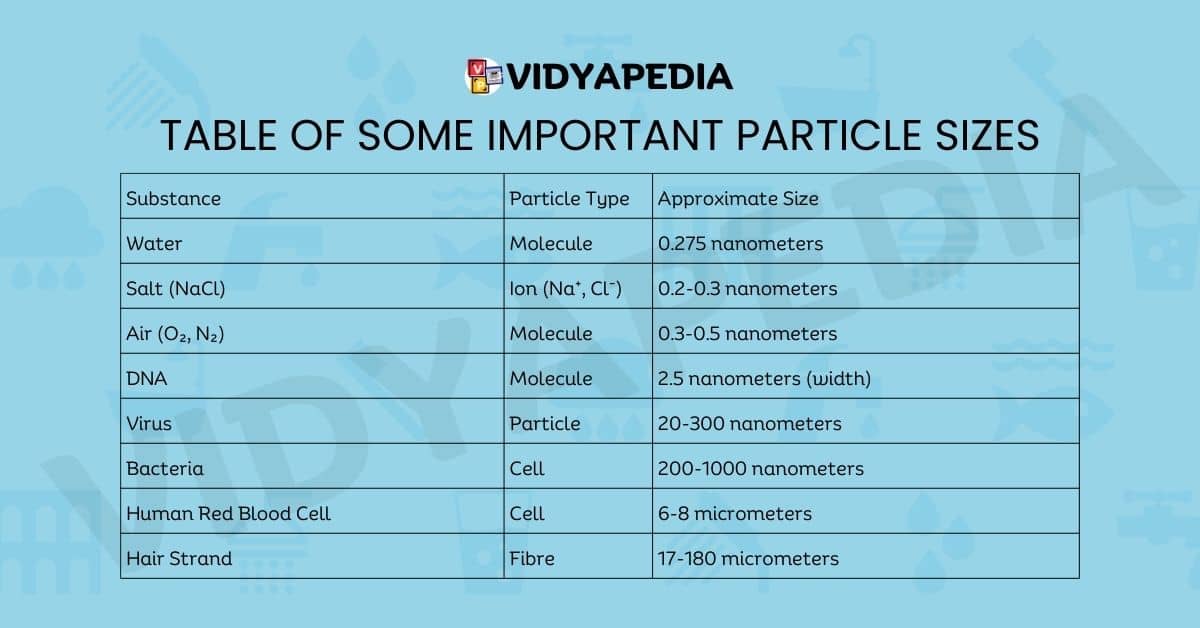
Table of Some Important Particle Sizes
| Substance | Particle Type | Approximate Size |
|---|---|---|
| Water | Molecule | 0.275 nanometers |
| Salt (NaCl) | Ion (Na⁺, Cl⁻) | 0.2-0.3 nanometers |
| Air (O₂, N₂) | Molecule | 0.3-0.5 nanometers |
| DNA | Molecule | 2.5 nanometers (width) |
| Virus | Particle | 20-300 nanometers |
| Bacteria | Cell | 200-1000 nanometers |
| Human Red Blood Cell | Cell | 6-8 micrometers |
| Hair Strand | Fibre | 17-180 micrometers |
F.A.Q’s
What does matter?
Matter includes everything in the universe, including living creatures and non-living things. It includes invisible concepts like thoughts and emotions, as well as tangible objects like air, water, and rocks.
Why does hot food’s scent travel further than cold food?
At higher temperatures, smell molecules spread more quickly, allowing the scent of hot food to go further than that of cold food.


