Introduction
Hello, students! Today, we’ll look at the interesting topic of the characteristics of particles of matter. Studying the properties of these particles allows us to understand the fundamental nature of the substances that surround us. Let’s look at the three main features of particles of matter:
- Particles of Matter Have Space Between Them
- Particles of Matter Are Continuously Moving
- Particles of Matter Attract Each Other
Read: Introduction of Matter
Particles of matter have space between them
Matter is made up of small particles that are not closely packed and instead have space between them. The amount of space varies with the condition of matter. Solids, for example, have very little space between particles, whereas liquids have more room, and gases have the greatest.
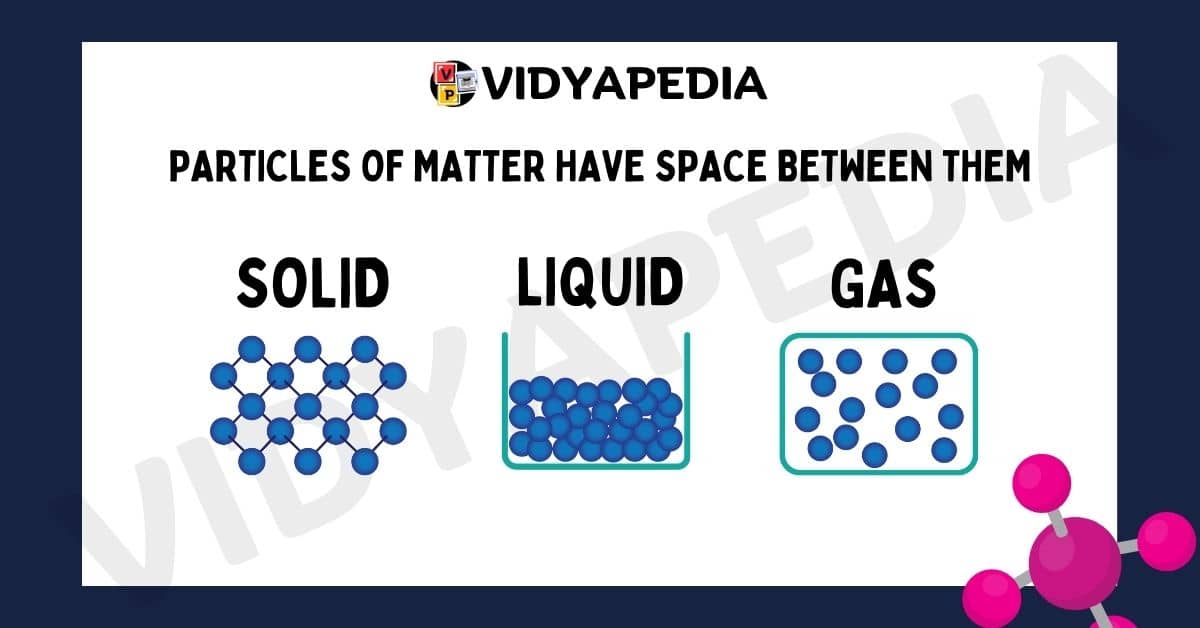
The amount of space between particles depends on the state of matter.
- Solid
- Liquid
- Gases
Solids: In solids, the particles are closely packed together with very little space between them. This close packing gives solids a definite shape and volume.
Liquids: In liquids, the particles are more spread out than in solids, but they are still relatively close to each other. This allows liquids to flow and take the shape of their container while maintaining a definite volume.
Gases: In gases, the particles are much farther apart compared to solids and liquids. This large amount of space between particles allows gases to expand and fill any container they are in, giving them neither a definite shape nor a definite volume.
Table Of Space Between Them
| State of Matter | Size of Space Between Particles | Volume | Shape |
|---|---|---|---|
| Solid | Very small | Definite | Definite |
| Liquid | Moderate | Definite | Takes shape of container |
| Gas | Large | Indefinite (fills container) | Indefinite |
Particles of Matter Are Continuously Moving
Particles in all states of matter are always in motion, and this motion is due to what we call kinetic energy. The nature and extent of this movement depend on the state of matter.
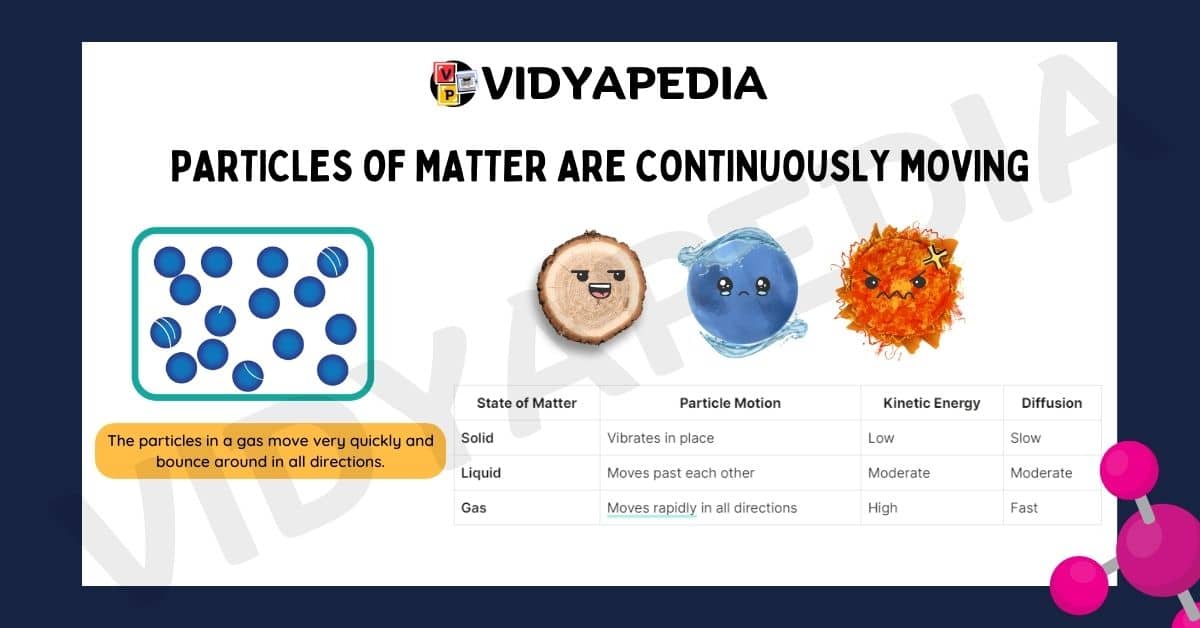
Solids: In solids, the particles vibrate in place. They are tightly packed and cannot move freely, but they constantly vibrate around their fixed positions.
Liquids: In liquids, the particles move more freely compared to solids. They can slide past one another, allowing liquids to flow and take the shape of their container.
Gases: In gases, the particles move rapidly and randomly in all directions. This high-speed movement allows gases to fill any container they are in, spreading out evenly throughout the space.
| State of Matter | Particle Motion | Kinetic Energy | Diffusion |
|---|---|---|---|
| Solid | Vibrates in place | Low | Slow |
| Liquid | Moves past each other | Moderate | Moderate |
| Gas | Moves rapidly in all directions | High | Fast |
That matter, particles naturally combine with one another. They attain this by entering the voids created by the particles. Diffusion is the independent mixing of particles of two distinct kinds of matter.
Read: Physical Properties of Matter
Particles of matter attract each other

Matter atoms are attracted to one another by forces. The characteristics of the particles and their separation from one another determine how strong these forces are. Strong attraction forces keep the particles tightly packed in solids. Because these forces are weaker in liquids, particles can move around while remaining near one another. Because of the extremely weak attraction forces in gases, particles can travel freely and a great distance apart.
Characteristics of particles of matter, class 9 notes
Frequently Asked Questions
What are the main features of particles of matter?
Particles of matter have three main characteristics: they have space between them, they are continuously moving, and they attract each other.
How does the space between particles vary with the state of matter?
The amount of space between particles depends on the state of matter. Solids have very little space between particles, liquids have more room, and gases have the greatest amount of space between particles
Why do particles of matter continuously move?
Particles of matter are in constant motion due to their possession of kinetic energy. This motion varies depending on the state of matter, with particles in solids vibrating in place, particles in liquids moving more freely past each other, and particles in gases moving rapidly and randomly in all directions.

