Understanding the different types of heterogeneous mixtures is fundamental in various scientific fields and everyday life. Mixtures can be broadly classified into homogeneous and heterogeneous types. This post will focus on heterogeneous mixtures, particularly solutions, suspensions, and colloidal mixtures. We will explore their definitions, characteristics, differences, and examples to enrich your knowledge of these fascinating combinations.
Types of Heterogeneous Mixtures
Heterogeneous mixtures are those where the components are not uniformly distributed, and the different parts can often be seen with the naked eye or under a microscope. This category includes three primary types of mixtures: solutions, suspensions, and colloids, each exhibiting unique properties and behaviours.
Solution: Its Definition, Characteristics, and Examples

Definition of Solution
A solution is a homogeneous mixture where one substance (the solute) is completely dissolved in another substance (the solvent). The resulting mixture has a uniform composition throughout.
Characteristics of Solutions
- Compositional Consistency: Solute particles are evenly distributed throughout the solvent.
- Particle Size: Solute particles are at the molecular or ionic level, typically less than 1 nanometer.
- Stability: Solutions are stable and do not separate over time.
- Transparency: Solutions allow light to pass through without scattering, making them clear and transparent.
Examples of Solutions
- Saltwater: Salt dissolved in water forms a clear, uniform solution.
- Soda: Carbonated beverages where carbon dioxide gas is dissolved in a liquid.
- Air: A solution of gases, primarily nitrogen and oxygen, with other trace gases evenly distributed.
Importance and Applications
Solutions play a critical role in various fields:
- Chemical Reactions: Solutions act as mediums for many chemical reactions.
- Biological Processes: In living organisms, solutions facilitate nutrient transport and waste removal.
- Industries: In industries such as pharmaceuticals and manufacturing, solutions are crucial for processes like drug formulation and material processing.
Suspension: Its Definition, Characteristics, and Examples

Definition of Suspension
A suspension is a heterogeneous mixture in which solid particles are dispersed in a liquid or gas but are not dissolved. These particles can be seen with the naked eye and will eventually settle out over time due to gravity.
Characteristics of Suspensions
- Compositional Diversity: Solid particles are not evenly distributed and can be seen with the naked eye.
- Particle Size: Particles are larger than those in solutions, typically greater than 1 micrometre.
- Stability: Suspensions are unstable and tend to separate over time.
- Light Scattering: Suspensions scatter light, a phenomenon known as the Tyndall effect.
Examples of Suspensions
- Muddy Water: Dirt particles suspended in water, giving it a turbid appearance.
- Flour in Water: Flour particles dispersed in water.
- Chalk in Water: Chalk particles mixed with water, forming a suspension.
Importance and Applications
Suspensions are commonly found in nature and everyday life:
- Environmental Systems: Suspensions like muddy water play a role in natural processes such as erosion and sediment transport.
- Medical Field: In medicine, suspensions are used in drugs that require a medium to deliver active ingredients.
- Food Industry: Many food products, such as soups and sauces, are suspensions that need to be well-mixed for consistency.
Colloidal Solutions: Their Definition, Characteristics, and Examples
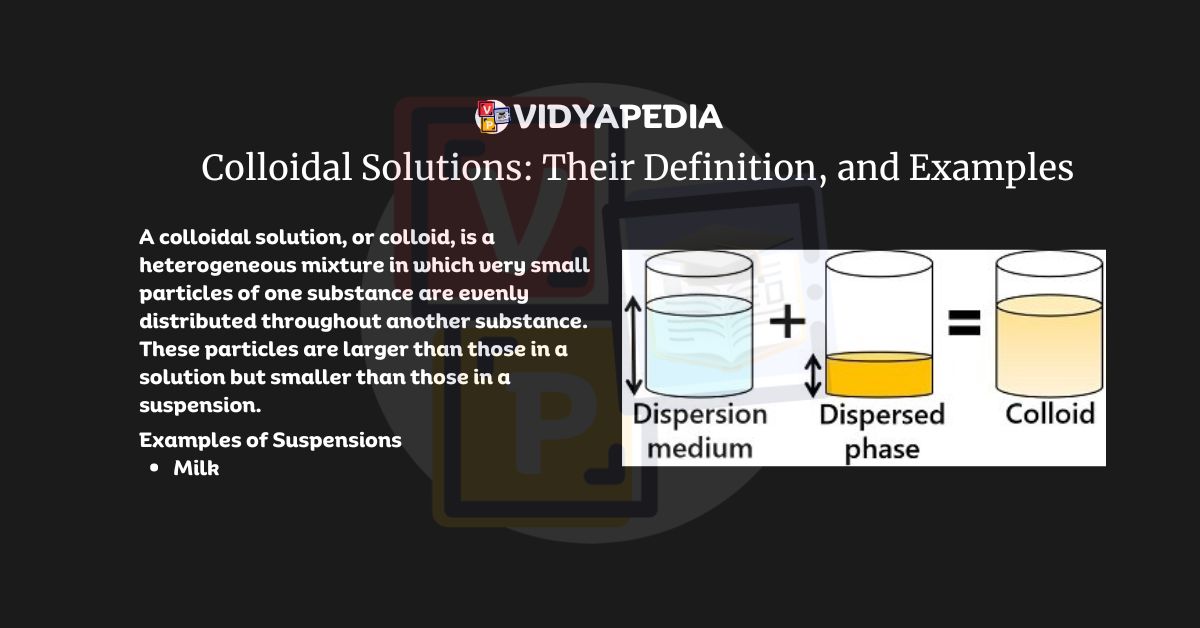
Definition of Colloidal Solution
A colloidal solution, or colloid, is a heterogeneous mixture in which very small particles of one substance are evenly distributed throughout another substance. These particles are larger than those in a solution but smaller than those in a suspension.
Characteristics of Colloidal Solutions
- Compositional Uniqueness: Colloids have finely divided particles dispersed in a continuous medium.
- Particle Size: Particles are between 1 nanometer and 1 micrometer.
- Stability: Colloids are generally stable and do not settle out over time.
- Light Scattering: Colloids exhibit the Tyndall effect, where particles scatter light.
Examples of Colloidal Solutions
- Milk: Fat globules disperse in water, giving it a milky appearance.
- Mayonnaise: Oil droplets suspended in water, forming a thick colloid.
- Fog: Tiny water droplets suspended in air, contributing to reduced visibility.
Importance and Applications
Colloids are significant in both natural and industrial contexts:
- Biological Systems: Many biological fluids are colloids, essential for bodily functions.
- Industrial Processes: Colloids are used in manufacturing processes such as paint and ink production.
- Daily Life: Everyday products like milk, creams, and sprays are colloidal mixtures.
Differences Between Solutions, Suspensions, and Colloids
Understanding the differences between these mixtures is crucial for their practical applications.
| Property | Solutions | Suspensions | Colloidal Solutions |
|---|---|---|---|
| Composition | Homogeneous mixture | Heterogeneous mixture | Heterogeneous mixture |
| Particle Size | < 1 nanometer | > 1 micrometer | 1 nanometer to 1 micrometer |
| Stability | Stable, particles do not settle | Unstable, particles settle over time | Stable, particles do not settle |
| Light Interaction | Transparent, no scattering | Tyndall effect, scatters light | Tyndall effect, scatters light |
| Visibility | Particles not visible | Particles visible | Particles not visible, seen under microscope |
| Examples | Saltwater, vinegar, air | Muddy water, flour in water, chalk in water | Milk, mayonnaise, fog, aerosols |
Frequently Asked Questions (FAQ)
What is the main difference between a solution and a suspension?
The main difference lies in the distribution and size of particles. Solutions have solute particles that are completely dissolved at the molecular level, making them homogeneous and transparent. Suspensions have larger particles that are not dissolved, making them heterogeneous and capable of settling over time.
How can you identify a colloidal solution?
A colloidal solution can be identified by the Tyndall effect, where light passing through the colloid is scattered by the dispersed particles. This effect makes the path of light visible, distinguishing colloids from true solutions.
Why do suspensions separate over time?
Suspensions separate over time due to the influence of gravity on the larger solid particles, which are heavy enough to settle at the bottom of the container, leading to phase separation and sedimentation.
Are all solutions transparent?
Yes, true solutions are transparent because the solute particles are at the molecular or ionic level, allowing light to pass through without scattering.
What role do solutions play in biological systems?
In biological systems, solutions facilitate crucial processes like nutrient transport, waste removal, hydration, and digestion, making them essential for maintaining life.


