The changing state of matter refers to the modifications that occur when matter transitions between its three primary states: solid, liquid, and gas. These changes occur as a result of temperature and pressure fluctuations, which have a direct effect on the substance’s kinetic energy and particle arrangement. Understanding these transitions is essential for comprehending many scientific events and everyday procedures.
Matter can change between three primary states: solid, liquid, and gas. Each state can transition to another under certain conditions:
- Solid to Liquid: When a solid is heated, it melts into a liquid. For example, ice melts to form water.
- Liquid to Gas: When a liquid is heated, it evaporates into a gas. For example, water boils to become steam.
- Gas to Liquid: When a gas is cooled, it condenses into a liquid. For example, steam cools down to form water droplets.
- Liquid to Solid: When a liquid is cooled, it freezes into a solid. For example, water freezes to form ice.
- Solid To Gas
- Gas To Solid
These above-changing states have the specified name. Based on the above introduction of kinetic energy and particle or matter arrangement, changing the state of matter can be classified as follows:
- Melting
- Freezing
- Evaporation
- Condensation
- Sublimation
- Deposition
Related Post: Fundamental States Of Matter And Their Properties
Characteristics of Particles of Matter
Introduction of Matter And Physical Nature Of Matter
Melting
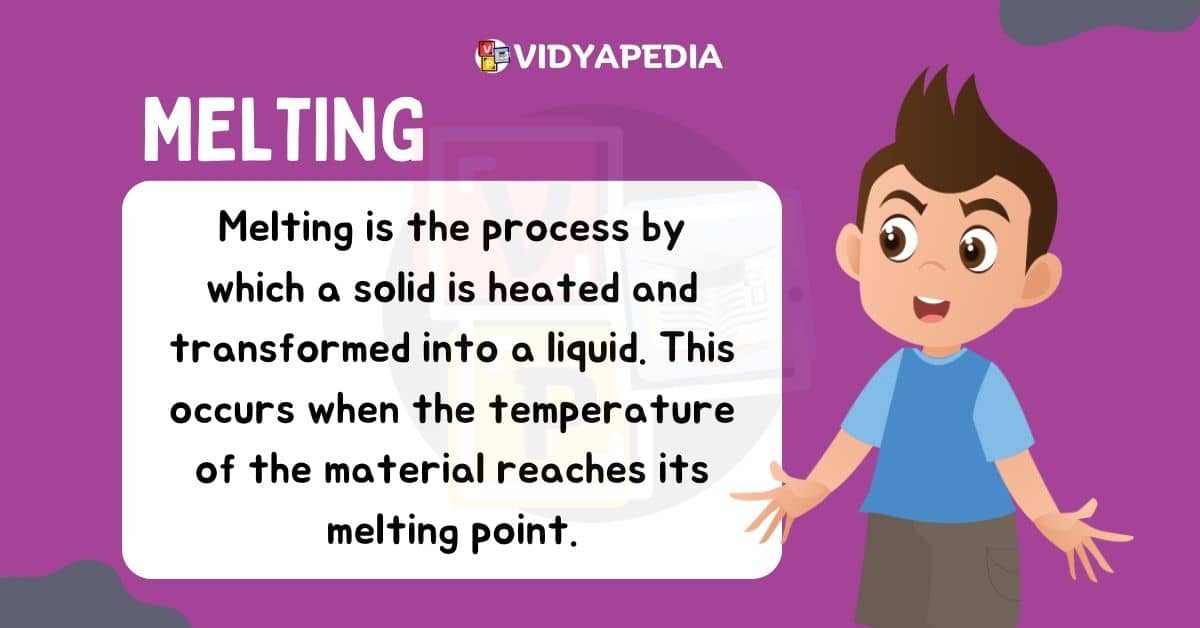

When the temperature of an object in a solid state increases and it transitions into a liquid state, this process is called melting. For example, when you heat a solid, its kinetic energy increases. The particles within the solid start to vibrate more vigorously, weakening the forces between them. As a result, the solid structure collapses, and it transforms into a liquid. The particles gain energy and become a liquid. This transition occurs at a specific temperature, known as the melting point.
Freezing
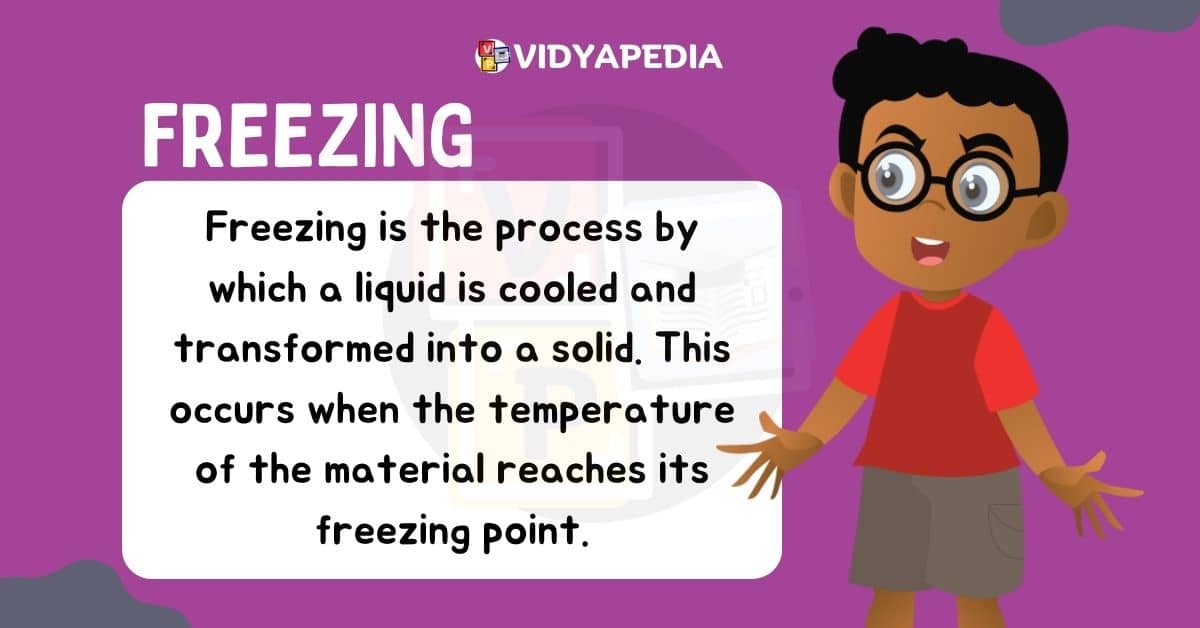

When a liquid leaves its state and transitions into a solid state due to a decrease in temperature, this process is called freezing. For example, if the temperature is decersed then kinetic energy will gradually decrease, causing the particles to come closer together. This aids in the formation of a solid structure. The freezing point is the specific temperature at which a liquid transforms into a solid.
Evaporation
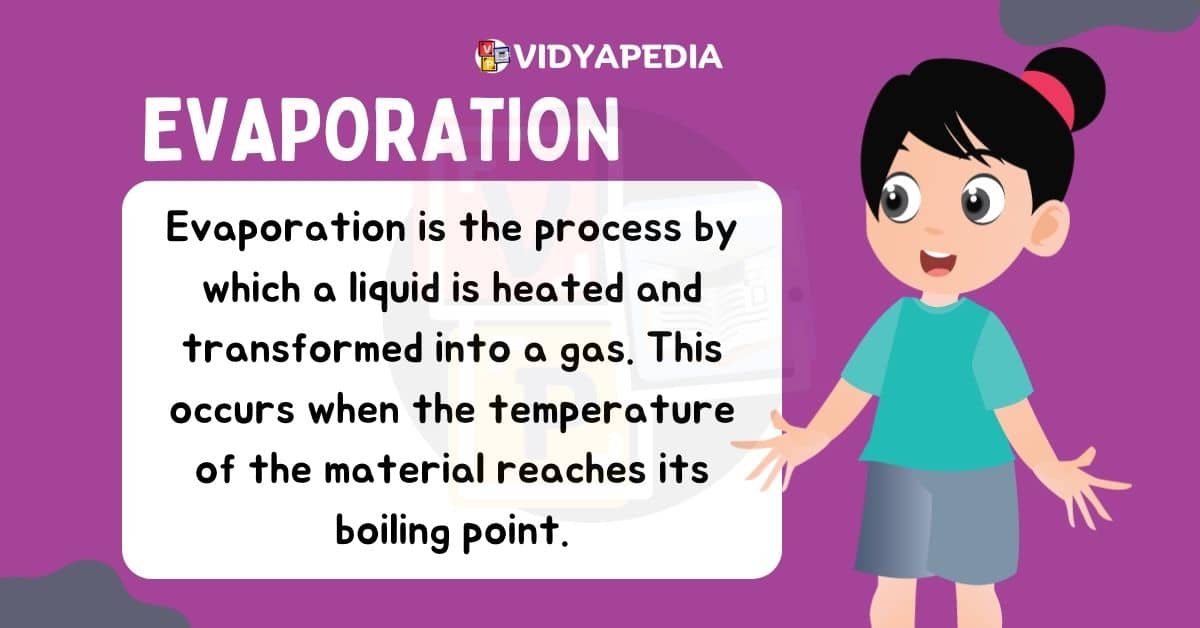
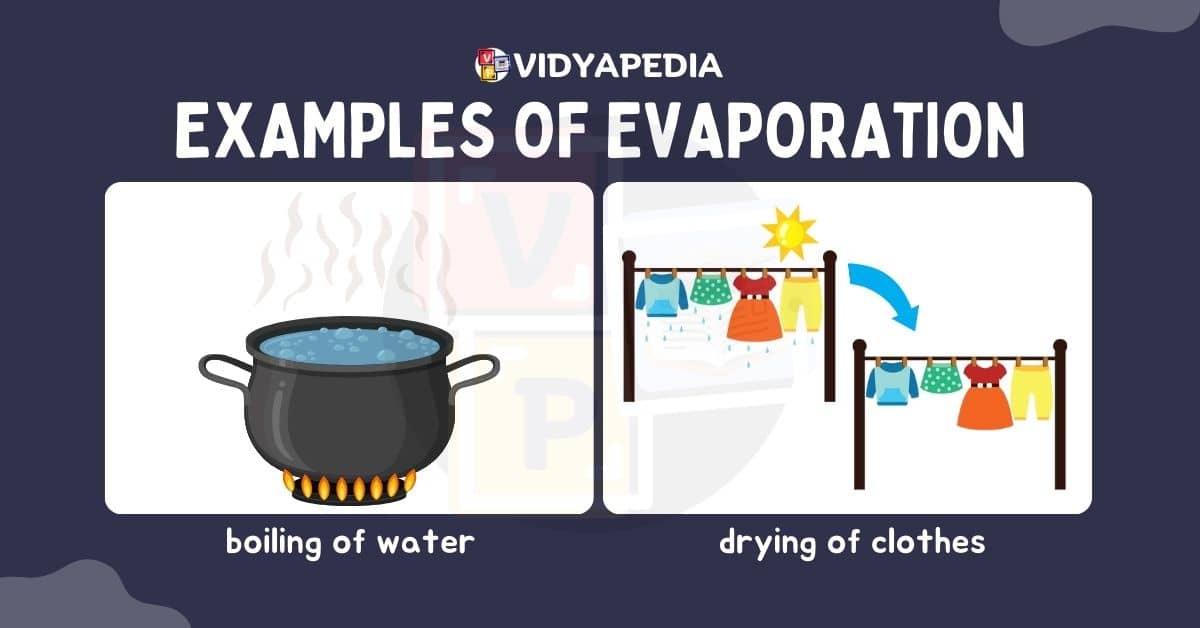
Evaporation is the process by which a liquid substance turns into a gas, which occurs largely near the liquid’s surface. Evaporation occurs when particles near the liquid’s surface obtain enough kinetic energy to escape into space as vapour. Higher temperatures make this process more active because greater heat energy gives the particles the energy they need to overcome intermolecular interactions and escape the liquid phase.
Condensation
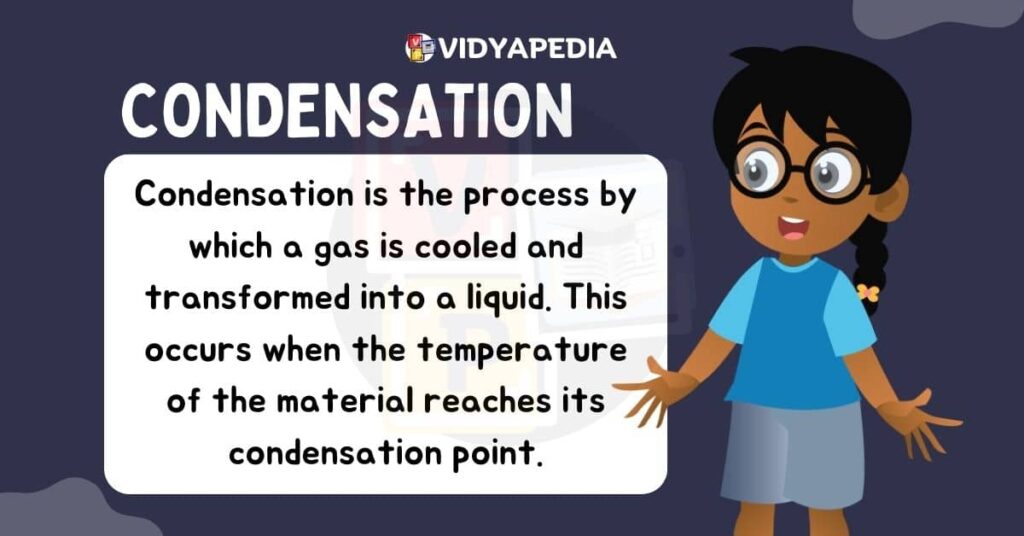
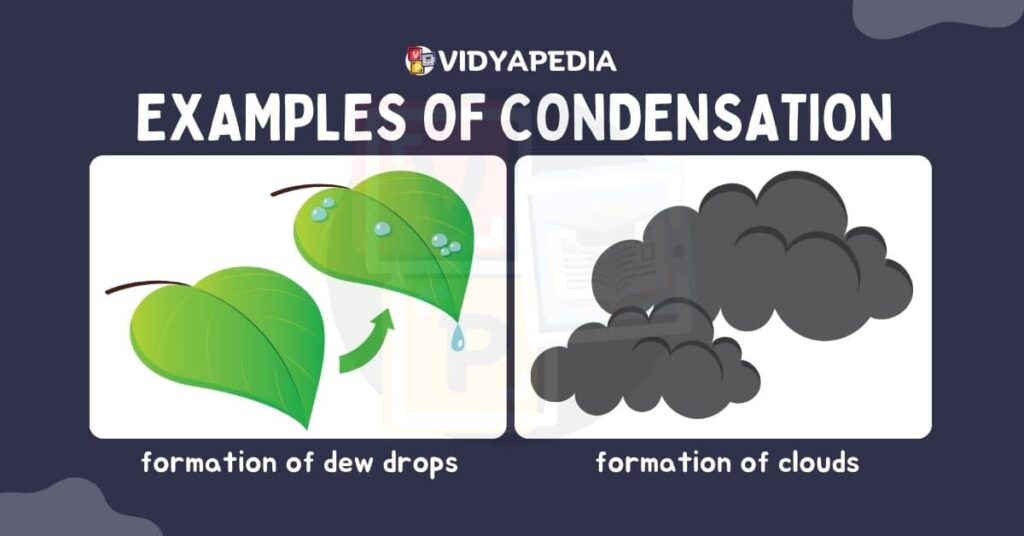
Condensation is the opposite of evaporation, in which gaseous particles lose energy and changes to the liquid state. This usually happens when vapour makes contact with a cooler surface, causing the particles to lose kinetic energy and cool down. As a result, they come closer and produce liquid droplets. Condensation is a typical event in cloud formation as water vapour cools and condenses into liquid water droplets.
Sublimation
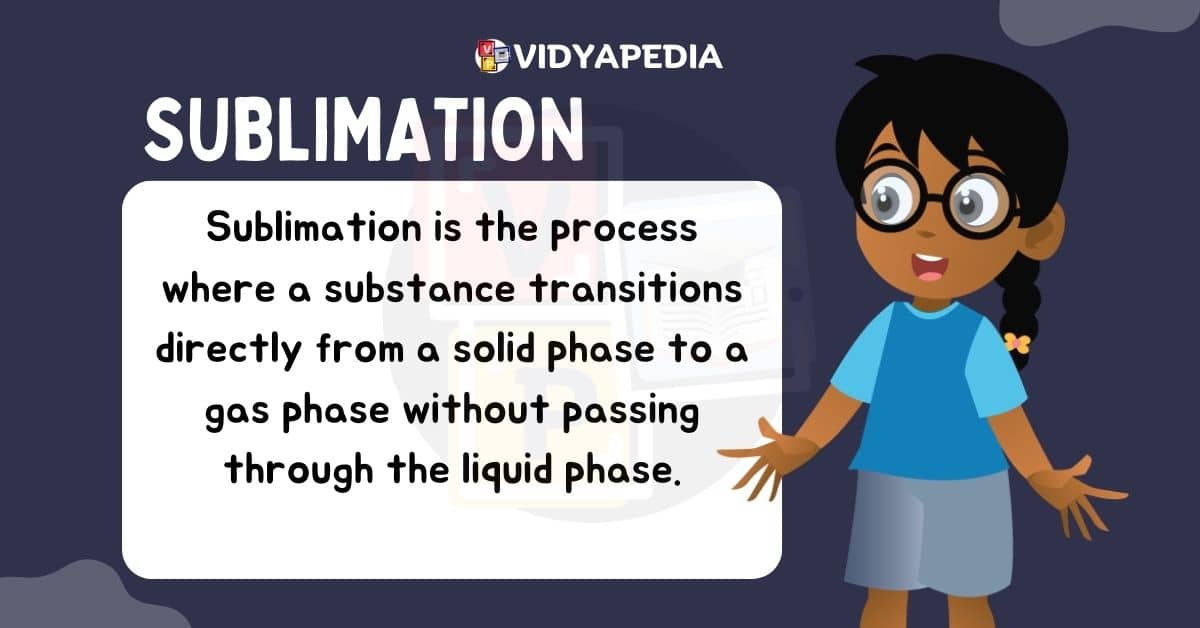
Sublimation is a direct change of state in which a solid substance transitions directly into a gaseous state without going through the liquid state. This happens when the solid’s vapour pressure exceeds air pressure at a specific temperature. The best Example Of Sublimation is the evaporation of dry ice (solid carbon dioxide) or the direct conversion of snow into water vapour in freezing circumstances.
Deposition
Deposition is the opposite of sublimation, in which a gaseous substance transforms directly into a solid state without going through the liquid phase. When a gas’s temperature drops below a certain level, its particles lose enough kinetic energy to form a solid structure. Deposition occurs in a variety of natural processes, including the creation of frost on surfaces and the crystallization of water vapour into snowflakes in freezing conditions.
Table Of Changing State Of Matter
| Name of Process | Changing State From | Changing State To | Temperature Increase/Decrease/Constant | Example |
|---|---|---|---|---|
| Melting | Solid | Liquid | Increase | Ice melting to form water |
| Freezing | Liquid | Solid | Decrease | Water freezing to form ice |
| Evaporation | Liquid | Gas | Increase | Water evaporating to form steam |
| Condensation | Gas | Liquid | Decrease | Steam condensing to form water droplets |
| Sublimation | Solid | Gas | Increase | Dry ice sublimating to form carbon dioxide gas |
| Deposition | Gas | Solid | Decrease | Frost forming from water vapor |
Frequently Asked Question
Can iron change its state of matter?
Yes, iron can change its state. It melts from solid to liquid at 1538°C and boils from liquid to gas at 2862°C. Cooling reverses these changes.
Can oxygen cahange its state of matter?
Yes, oxygen can change its state. It liquefies at -183°C and solidifies at -218°C under standard atmospheric pressure.
Can the state of matter be changed by decreasing pressure?
Yes, decreasing pressure can change the state of matter because it affects the intermolecular forces holding the particles together.